

- #GRAPHPAD PRISM 4 COMPATIBILITY MANUAL#
- #GRAPHPAD PRISM 4 COMPATIBILITY SOFTWARE#
- #GRAPHPAD PRISM 4 COMPATIBILITY SERIES#
Stata allows users to have control over data, variables and also statistical compilation of groups.
#GRAPHPAD PRISM 4 COMPATIBILITY SERIES#
There are a lot of statistical features, stretching from descriptive analysis, cross tabulation analysis to more advanced techniques like structural equation modeling, probability models, survival analysis, time series and multilevel models. STATA is compatible with Excel files (.xls. Stata features includes, Graphical user interface (GUI) or simply, point-and-click interface accompanied with an option for command line interface (CLI) which is quick, authentic and easy to use. The name Stata is a syllabic abbreviation of the words statistics and data and was released in 1985 and then the graphical user interface option in 2003.
#GRAPHPAD PRISM 4 COMPATIBILITY SOFTWARE#
It remains the most powerful software available in the analytics space. Stata can be termed as the policy statistical software common to institutions, including international organizations like the United Nations, governments and academicians for Public health, Economics, Social Work and Medicine. Stata is a complete toolbox that provides a data management capability, data analysis and a colorful graphical interface. Let’s look at the top 10 statistical tools used in medical research by scientists, physicians and industry R&D professionals. These tools handle the end-to-end processes of collecting, organizing, analyzing and interpreting statistical data. These tools get the job done in similar ways, but the differences lie in ease of use and presentation as differences in licensing (proprietary or not), interface (point and click or command line) and cost (free or paid). There are a vast universe of statistical tools used in medical research.
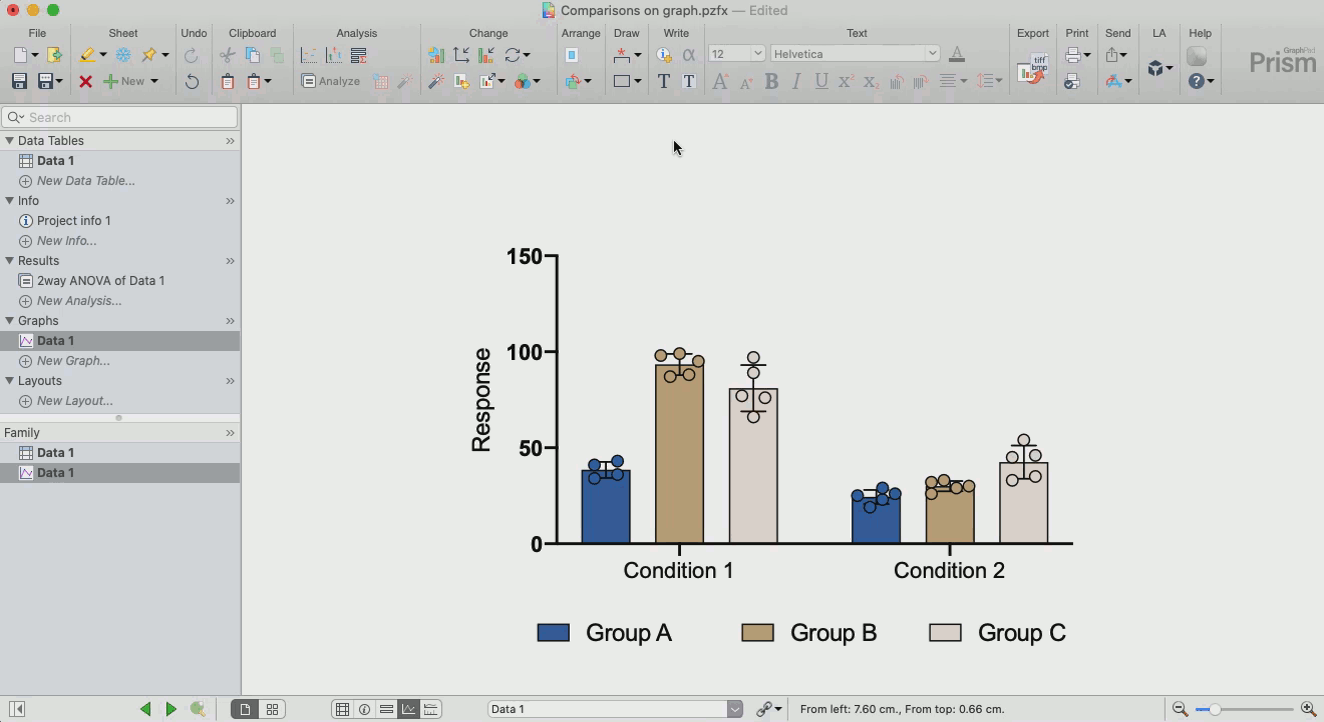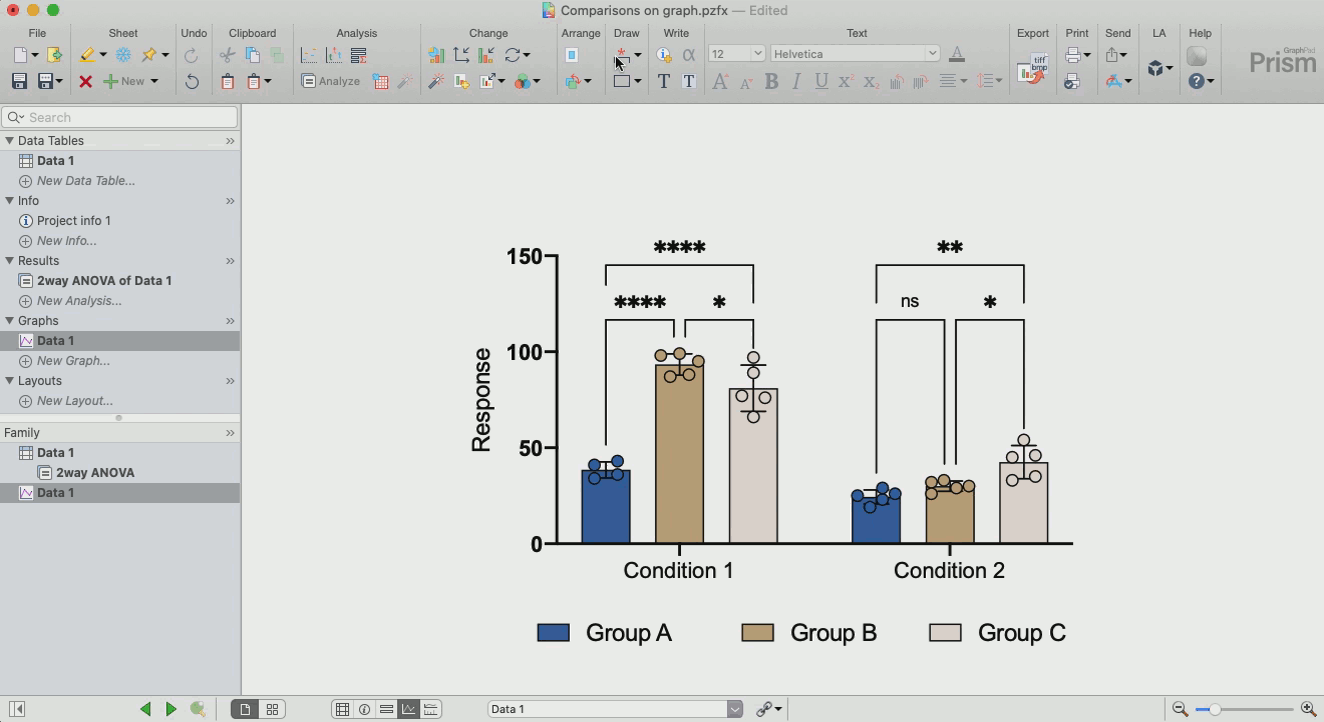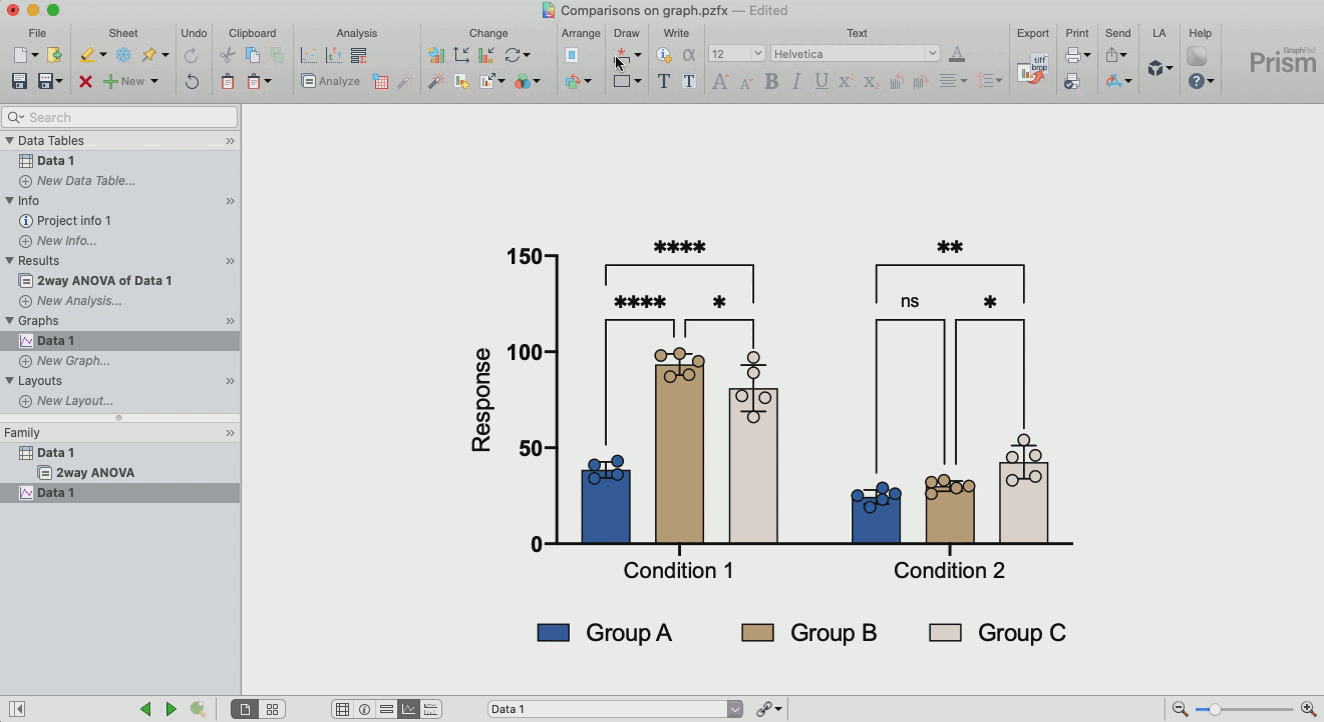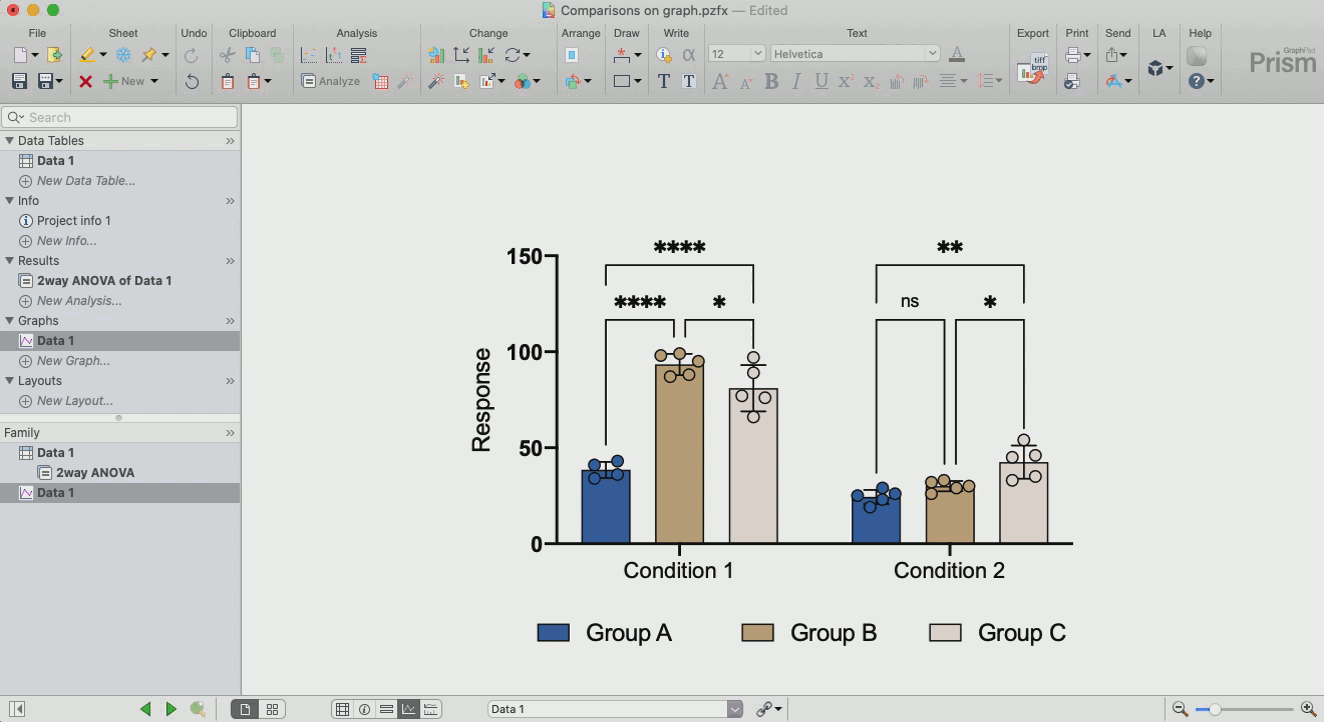
How to make sense out of the data (interpretation of results)
What to do with the data (choice of estimation test) Understand the origin of the data, by way of the research objectives The process can be broken down into 3 clear steps: However, since data in itself is never normally distributed or perfect, it becomes important to apportion a percentage of 0.01 (1%) as the level of significance or margin of error or probability that the result will produce an error, though slight but gets better as the benchmark approaches 100%, in other words, 0.001 (0.1%) or 0.0001 (0.01%).ĭata in itself consisting of an admixture of numeric, string and alpha numerical points can appear intimidating but the analysis of data does not have to be always complex. In testing research hypothesis, the assumption is based on 100% correctness. Validation parameters must be more stringent. In the medical research field, stretching from systematic reviews, meta-analysis and clinical trials, exactness and precision is paramount.

Today, due to the gradual advancement in technology, statistical tools are being used in medical research for greater efficiency and accuracy. This opened up gaps for human error and greater cost of carrying out research especially when the data was large by say, over a 1000 field observations.
#GRAPHPAD PRISM 4 COMPATIBILITY MANUAL#
There was a time when validating experiments through data was done fully using manual computation. Kolabtree freelance statistician Kingsley Ukwuoma writes about the top statistical tools used in medical research and clinical data analysis.


 0 kommentar(er)
0 kommentar(er)
